

|
TABLE 3A. Prophylaxis for first episode of opportunistic disease in
HIV-infected infants and children
|
|
|||
| Pathogen | Indication | First choice | Alternatives |
| I. Strongly recommended as standard of care | |||
| Pneumocystis carinii* |
HIV-infected or HIV-indeterminate infants aged 1–12 mo;
HIV-infected children aged 1–5 yr with CD4+ count <500/µL or CD4+ percentage <15%;
HIV-infected children aged 6–12 yr with CD4+ count <200/µL or CD4+ percentage <15%
|
Trimethoprim-sulfamethoxazole (TMP-SMZ), 150/750 mg/m2 /d in 2
divided doses po t.i.w. on consecutive days (AII)
Acceptable alternative dosage schedules: (AII)
Single dose po t.i.w. on consecutive days; 2 divided doses po q.d.; 2 divided doses po t.i.w. on alternate days
|
Aerosolized pentamidine (children aged >5 yr), 300 mg q.m. via
Respirgard II™ nebulizer (CIII); dapsone (children aged >1
mo), 2 mg/kg (max 100 mg) po q.d. (CIII); IV pentamidine, 4 mg/kg every 2–4
weeks (CIII)
|
| Mycobacterium tuberculosis: | |||
| Isoniazid-sensitive | Isoniazid- TST reaction >5mm or prior positive TST result without treatment or contact with case of active tuberculosis | Isoniazid 10–15 mg/kg (max 300 mg) po or im q.d. x 12 mo (AI) or 20–30 mg/kg (max 900 mg) po b.i.w. x 12 mo (BIII) | Rifampin, 10–20 mg/kg (max 600 mg) po or iv q.d. x12 mo (BII) |
| Isoniazid-resistant | Same as above; high probability of exposure to isoniazid-resistant tuberculosis | Isoniazid 10–15 mg/kg (max 300 mg) po or im q.d. x 12 mo (AI) or 20–30 mg/kg (max 900 mg) po b.i.w. x 12 mo (BIII) | Uncertain |
| Multidrug (isoniazid and rifampin)-resistant | Same as above; high probability of exposure to multidrug-resistant tuberculosis | Choice of drug requires consultation with public health authorities | None |
| Mycobacterium avium complex | For children aged >6 yrs, CD4+ count <50/µL ; aged 2–6 yrs, CD4+ count <75/µL ; aged 1–2 yrs, CD4+ count <500/µL ; aged <1 yr, CD4+ count <750/µL | Clarithromycin, 7.5 mg/kg (max 500 mg) po b.i.d. (AII), or azithromycin, 20 mg/kg (max 1,200 mg) po q.w. (AII) | Children aged >6 yrs, rifabutin, 300 mg po q.d. (BI); children aged <6 yrs, 5 mg/kg po q.d. when suspension becomes available (BI); azithromycin, 5 mg/kg (max 250 mg) po q.d. (AII) |
|
Varicella zoster virus
|
Significant exposure to varicella with no history of chickenpox or shingles | Varicella zoster immune globulin (VZIG), 1 vial (1.25 mL)/10 kg (max 5 vials) im, administered <96 hrs after exposure, ideally within 48 hrs (AII) | None |
| Vaccine-preventable pathogens § | HIV exposure/infection | Routine immunizations (see Figure) | None |
| II. Generally recommended | |||
| Toxoplasma gondii ¶ | IgG antibody to Toxoplasma and severe immunosuppression | TMP-SMZ, 150/750 mg/m2/d in 2 divided doses po q.d. (BIII) | Dapsone (children aged >1 mo), 2 mg/kg or 15 mg/m2 (max 25 mg) po q.d. plus pyrimethamine, 1 mg/kg po q.d. plus leucovorin, 5 mg po every 3 days (BIII) |
| III. Not recommended for most patients; indicated for use only in unusual circumstances | |||
| Invasive bacterial infections** | Hypogammaglobulinemia | IVIG (400 mg/kg/q.m.) (AI) | None |
| Candida species | Severe immunosuppression | Nystatin (100,000 U/mL), 4–6 mL po q 6 h (CIII) or topical clotrimazole, 10 mg po 5x/d (CIII) | None |
| Cryptococcus neoformans | Severe immunosuppression | Fluconazole, 3–6 mg/kg po q.d. (CII) | Itraconazole, 2–5 mg/kg po q 12–24 h (CIII) |
| Histoplasma capsulatum | Severe immunosuppression, endemic geographic area | Itraconazole, 2–5 mg/kg po q 12–24 h (CII) | None |
|
Cytomegalovirus
(CMV) |
CMV antibody positivity and severe immunosuppression | severe immunosuppression ganciclovir under investigation | None |
The Respirgard II™ nebulizer is manufactured by Marquest, Englewood, CO.
Letters and Roman numerals in parentheses after regimens indicate the strength of the recommendation and the quality of the evidence supporting it (see text).
* The efficacy of parenteral pentamidine (e.g., 4 mg/kg/month) is controversial. TMP-SMZ, dapsone-pyrimethamine, and possibly dapsone alone appear to protect against toxoplasmosis, although data have not been prospectively collected. Daily TMP-SMZ reduces the frequency of some bacterial infections. Patients receiving therapy for toxoplasmosis with sulfadiazine-pyrimethamine are protected against Pneumocystis carinii pneumonia (PCP) and do not need TMP-SMZ.
![]() Children
routinely being administered intravenous immune globulin (IVIG) should receive
VZIG if the last dose of IVIG was administered >21 days before exposure.
Children
routinely being administered intravenous immune globulin (IVIG) should receive
VZIG if the last dose of IVIG was administered >21 days before exposure.
§ HIV-infected and HIV-exposed children should be immunized according to the following childhood immunization schedule (Figure), which has been adapted from the January–December 1997 schedule recommended for immunocompetent children by the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians. This schedule differs from that for immunocompetent children in that IPV replaces OPV, vaccination against S. pneumoniae (AII) and influenza (BIII) should be offered, and vaccination against varicella is contraindicated (EIII). MMR should not be administered to severely immunocompromised children (DIII). Once an HIV-exposed child is determined not to be HIV infected, the schedule for immunocompetent children applies.
¶ Protection against Toxoplasma is provided by the preferred antipneumocystis regimens. Pyrimethamine alone probably provides little, if any, protection. For definition of severe immunosuppression, see Table 1.
** Respiratory syncytial virus (RSV) IVIG may be substituted for IVIG during the RSV season.
![]()
![]() Data on
oral ganciclovir are still being evaluated; durability of effect is unclear.
Acyclovir is not protective against CMV.
Data on
oral ganciclovir are still being evaluated; durability of effect is unclear.
Acyclovir is not protective against CMV.
Immunization Schedule for HIV-infected children
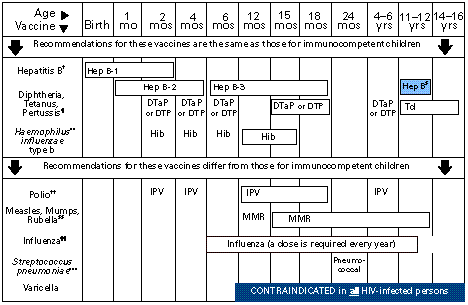
*Vaccines are listed under the routinely recommended ages. Bars indicate range of acceptable ages for vaccination. Shaded bars indicate catch-up vaccination: at 11–12 years of age, hepatitis B vaccine should be administered to children not previously vaccinated.
![]() Infants
born to HBsAg-negative mothers should receive 2.5µg of Merck vaccine
(Recombivax HB® ) or 10µg of SmithKline Beecham (SB) vaccine
(Engerix-B® ). The 2nd dose should be administered >1 mo after the
1st dose.
Infants
born to HBsAg-negative mothers should receive 2.5µg of Merck vaccine
(Recombivax HB® ) or 10µg of SmithKline Beecham (SB) vaccine
(Engerix-B® ). The 2nd dose should be administered >1 mo after the
1st dose.
Infants born to HBsAg-positive mothers should receive 0.5 mL of hepatitis
B immune globulin (HBIG) within 12 hrs of birth and either 5µg of Merck
vaccine (Recombivax HB®) or 10µg of SB vaccine (Engerix-B®)
at a separate site. The 2nd dose is recommended at 1–2 mos of age and
the 3rd dose at 6 mos of age.
Infants born to mothers whose HBsAg status is unknown should receive
either 5µg of Merck vaccine (Recombivax HB® ) or 10µg of SB
vaccine (Engerix-B® ) within 12 hrs of birth. The 2nd dose of vaccine
is recommended at 1 mo of age and the 3rd dose at 6 mos of age. Blood should
be drawn at the time of delivery to determine the mother's HBsAg status;
if it is positive, the infant should receive HBIG as soon as possible (no
later than 1 wk of age). The dosage and timing of subsequent vaccine doses
should be based upon the mother's HBsAg status.
§ Children and adolescents who have not been vaccinated against hepatitis B in infancy may begin the series during any childhood visit. Those who have not previously received 3 doses of hepatitis B vaccine should initiate or complete the series during the 11- to 12-year–old visit. The 2nd dose should be administered at least 1 mo after the 1st dose, and the 3rd dose should be administered at least 4 mos after the 1st dose and at least 2 mos after the 2nd dose.
¶ DTaP (diphtheria and tetanus toxoids and acellular pertussis vaccine) is the preferred vaccine for all doses in the vaccination series, including completion of the series in children who have received >1 dose of whole-cell DTP vaccine. Whole-cell DTP is an acceptable alternative to DTaP. The 4th dose of DTaP may be administered as early as 12 mos of age, provided 6 mos have elapsed since the 3rd dose, and if the child is considered unlikely to return at 15–18 mos of age. Td (tetanus and diphtheria toxoids, adsorbed, for adult use) is recommended at 11–12 yrs of age if at least 5 yrs have elapsed since the last dose of DTP, DTaP, or DT. Subsequent routine Td boosters are recommended every 10 yrs.
**Three H. influenzae type b (Hib) conjugate vaccines are licensed for infant use. If PRP-OMP (PedvaxHIB® [Merck]) is administered at 2 and 4 mos of age, a dose at 6 mos is not required. After the primary series has been completed, any Hib conjugate vaccine may be used as a booster.
![]()
![]() Inactivated
poliovirus vaccine (IPV) is the only polio vaccine recommended for HIV-infected
persons and their household contacts. Although the third dose of IPV is generally
administered at 12–18 months, the third dose of IPV has been approved
to be administered as early as 6 months of age. Oral poliovirus vaccine (OPV)
should NOT be administered to HIV-infected persons or their household contacts.
Inactivated
poliovirus vaccine (IPV) is the only polio vaccine recommended for HIV-infected
persons and their household contacts. Although the third dose of IPV is generally
administered at 12–18 months, the third dose of IPV has been approved
to be administered as early as 6 months of age. Oral poliovirus vaccine (OPV)
should NOT be administered to HIV-infected persons or their household contacts.
§§ MMR should not be administered to severely immunocompromised children. HIV-infected children without severe immunosuppression should routinely receive their first dose of MMR as soon as possible upon reaching the first birthday. Consideration should be given to administering the second dose of MMR vaccine as soon as one month (i.e., minimum 28 days) after the first dose, rather than waiting until school entry.
¶¶ Influenza virus vaccine should be administered to all HIV-infected children >6 months of age each year. Children aged 6 months–8 years who are receiving influenza vaccine for the first time should receive two doses of split virus vaccine separated by at least one month. In subsequent years, a single dose of vaccine (split virus for persons <12 years of age, whole or split virus for persons >12 years of age) should be administered each year. The dose of vaccine for children aged 6–35 months is 0.25 mL; the dose for children aged >3 years is 0.5 mL.
***The 23-valent pneumococcal vaccine should be administered to HIV-infected children at 24 months of age. Revaccination should generally be offered to HIV-infected children vaccinated 3–5 years (children aged <10 years) or >5 years (children aged >10 years) earlier.
TABLE 3B. Prophylaxis for recurrence of opportunistic disease (after chemotherapy for acute disease) in HIV-infected infants and children
|
|
|||
| Pathogen | Indication | First choice | Alternative |
| I. Recommended for life as standard of care | |||
| Pneumocystis carinii | Prior P. carinii pneumonia |
TMP-SMZ, 150/750 mg/m2 /d in 2 divided doses po t.i.w. on consecutive
days (AII)
Single dose po t.i.w. on consecutive days; 2 divided doses po q.d.; 2 divided doses po t.i.w. on alternate days |
Aerosolized pentamidine (children aged >5 yrs), 300 mg q.m. via Respirgard II™ nebulizer (CIII); dapsone (children aged >1 mo), 2 mg/kg (max 100 mg) po q.d. (CIII); iv pentamidine, 4 mg/kg every 2–4 weeks (CIII) |
| Toxoplasma gondii* | Prior toxoplasmic encephalitis | Sulfadiazine, 85–120 mg/kg/d in 2–4 divided doses po q.d. plus pyrimethamine, 1 mg/kg or 15 mg/m2 (max 25 mg) po q.d. plus leucovorin, 5 mg po every 3 days (AI) | Clindamycin, 20–30 mg/kg/d in 4 divided doses po q.d. 1 mg/kg po q.d. plus leucovorin, 5 mg po every 3 days (BI) |
| Mycobacterium avium complex | Prior disease | Clarithromycin, 7.5 mg/kg (max 500 mg) po b.i.d. (AII) plus at least one of the following: ethambutol, 15 mg/kg (max 900 mg) po q.d. (AII); rifabutin, 5 mg/kg (max 300 mg) po q.d. (AII) | |
| Cryptococcus neoformans | Documented disease | Fluconazole, 3–6 mg/kg po q.d. (AII) | Itraconazole, 2–5 mg/kg po q 24–12h (BII); amphotericin B, 0.5–1.5 mg/kg iv 1–3x/week (AI) |
| Histoplasma capsulatum | Documented disease | Itraconazole, 2–5 mg/kg po q 12–48h (AIII) | Fluconazole, 3–6 mg/kg po q.d. (CIII); amphotericin B, 1.0 mg/kg iv q.w. AIII) |
| Coccidioides immitis | Documented disease | Fluconazole, 6 mg/kg po q.d. (AIII) | Amphotericin B, 1.0 mg/kg iv q.w. (AII) |
| Cytomegalovirus | Prior end-organ disease | Ganciclovir, 5 mg/kg iv q.d.; or foscarnet, 90–120 mg/kg iv q.d. (AI); or (for retinitis) ganciclovir sustained-release implant (AI) | |
|
Salmonella species (non-typhi)
|
Bacteremia | TMP-SMZ, 150/750 mg/m2 in 2 divided doses po q.d. for several months (CIII) | Antibiotic chemoprophylaxis with another active agent (CIII) |
| II. Recommended only if subsequent episodes are frequent or severe | |||
| Invasive bacterial infections § | >2 infections in 1-year period | TMP-SMZ, 150/750 mg/m2 in 2 divided doses po q.d. (BI); or IVIG, 400 mg/kg q.m. (BI) | Antibiotic chemoprophylaxis with another active agent (BIII) |
| Herpes simplex virus | Frequent/severe recurrences | Acyclovir, 80 mg/kg/d in 3–4 divided doses po q.d. (AII) | |
| Candida species | Frequent/severe recurrences | Fluconazole, 3–6 mg/kg po q.d. (AII); or ketoconazole, 5–10 mg/kg po q 24–12h (CIII) | |
The Respirgard II™ nebulizer is manufactured by Marquest, Englewood, CO.
Letters and Roman numerals in parentheses after regimens indicate the strength of the recommendations and the quality of the evidence supporting it (see text).
* Only pyrimethamine plus sulfadiazine confers protection against PCP as well as toxoplasmosis. Although the clindamycin plus pyrimethamine regimen is the preferred alternative in adults, it has not been tested in children. However, these drugs are safe and are used for other infections.
![]() Drug
should be determined by susceptibilities of the organism isolated. Alternatives
to TMP-SMZ include ampicillin, chloramphenicol, or ciprofloxacin. However,
ciprofloxacin is not approved for use in persons aged <18 years; therefore,
it should be used in children with caution and only if no alternatives exist.
Drug
should be determined by susceptibilities of the organism isolated. Alternatives
to TMP-SMZ include ampicillin, chloramphenicol, or ciprofloxacin. However,
ciprofloxacin is not approved for use in persons aged <18 years; therefore,
it should be used in children with caution and only if no alternatives exist.
§ Antimicrobial prophylaxis should be chosen based on the microorganism and antibiotic sensitivities. TMP-SMZ, if used, should be administered daily. Providers should be cautious about using antibiotics solely for this purpose because of the potential for development of drug-resistant microorganisms. IVIG may not provide additional benefit to children receiving daily TMP-SMZ. Choice of antibiotic prophylaxis vs. IVIG should also involve consideration of adherence, ease of intravenous access, and cost. If IVIG is used, RSV-IVIG may be substituted for IVIG during the RSV season.
Go to the USPHS/IDSA Guidelines Menu
Go to the Opportunistic Infections Menu
Go to the HIVpositive.us Main Menu